Chicken growth is influenced by a multitude of factors. While nutrition is the most crucial factor influencing the growth curve in broiler chickens, this article aims to provide information about other factors that can affect the growth of chickens during fattening. These factors can be managed by farmers to achieve optimal chicken growth.
The optimal growth of chickens is the primary objective of farmers, who can influence the conditions at the farm. However, certain factors in the chicken growth curve take effect earlier than the fattening period. For example, the age of a breeder flock can significantly influence the growth of its offspring. Chickens hatched from an older parent flock (59 weeks old) had a 13% and 4% higher weight at 21 and 41 days of age, respectively, compared to those hatched from a 29-week-old parent flock. This effect is due to the larger eggs laid by the older breeder flock (Ulmer-Franco et al., 2010). Subsequently, incubation conditions are of paramount importance at the hatchery level to ensure optimal chicken growth. A comparison of three temperatures (36.5°C, 37.6°C, and 38.7°C) applied in hatchery cabinets over five days of incubation has been found to significantly influence the live weight of chickens at 1, 21, 35, and 44 days of age. The optimal temperature for chicken growth was found to be 38.7 °C (Hulet et al., 2007). The basis for chicken growth is established before fattening.
Chick quality
For farmers, it is important to designate clear parameters for assessing the quality of day-old chicks in order to make adjustments in flock management to adhere to prescribed growth curves during the fattening process. The optimal hatching egg weight is approximately 60 grams, as this weight is conducive to optimal hatchability. Additionally, hatching egg weight is a determining factor of the chick’s weight immediately after hatching and subsequently during fattening (Iqbal et al., 2016). Willemsen et al. (2008) compared easily measurable parameters of chick quality (chick body weight, chick length, shank length, and toe length) and their predictive value for future chicken growth. The body weight at one day of age exhibited the second highest predictive value. The weight at seven days of age proved to be the most accurate predictor of chicken growth. This conclusion was also reached by Tona et al. (2004). The weight of week-old broilers appears to be a reliable indicator of growth curve compliance during fattening. According to the established guidelines, the hatched live body weight of the main hybrids Cobb 500 and Ross 308 at seven days of age is 202 g and 213 g, respectively. Therefore, achieving this weight indicates the presence of optimal chicken quality and a high potential for optimal growth.
Temperature
Fluctuations in the growth curve often reflect fluctuations in environmental conditions. Temperature is the most significant environmental factor on growth performance, followed by ventilation rate (Baracho et al., 2019). Meta-analyses performed by Liu et al. (2020) showed negative effects of heat stress in the second half of the growing period on the performance of chickens. Heat-stressed chickens in studies included in meta-analyses were exposed to various temperatures from 27.8 °C to 38 °C in continuous or cyclical manners. Heat stress decreased feed intake by 98 g and body weight gain by 151 g, and it also worsened the feed conversion ratio by an average of 0.17 in heat stressed chickens in the collected studies (Liu et al., 2020). To ensure optimal growth, farmers can offset environmental conditions by adjusting stocking density. In cases of prominent heat stress, it can be beneficial to decrease stocking density in the barn. Higher stocking density promotes heat production, thus potentially leading to increased heat stress. In a study by Son et al. (2022), 16, 18, 21, 23, and 26 chickens per square meter were exposed to a temperature of 33 °C for seven days. During the heat stress period, the highest body weight gain was observed at a density of 18 chickens per square meter, and weight gain decreased linearly with increasing chicken density. At the highest density (26 chickens/m2), the lowest body weight gain (459 g) was observed, in comparison to the density of 18 chickens/m2, where 666 g of weight gain was observed during the heat stress period. The feed conversion ratio worsened by 0.5 from 18 chickens/m2 to 26 chickens/m2. Therefore, while increasing stock density can result in higher yields, the resulting heat and the stress from it can have a deleterious effect on growth rates. Farmers adjust for this by decreasing stocking density in summer months in order to optimize growth curves. They also employ tunnel ventilation as a reactionary measure to acutely alleviate heat stress and stabilize the development of their flocks. The optimal air velocity in summer to ensure the best growth of chickens is between 1.5 and 2 m/s (Yahav et al., 2001). The objective of flock management during the summer months is to ensure optimal temperature conditions within the barn in order to maintain homeostasis in the chickens.
Litter quality
It is important to note that the litter at the end of the fattening period can also negatively influence the chicken growth. This is particularly true when the litter quality is poor, as the consequent increase in ammonium levels can stunt bird development. It is therefore crucial to maintain ammonium levels below 25 ppm (Yi et al., 2016). However, at the end of the fattening period, it is not uncommon for these levels to exceed 50 ppm (Miles et al., 2004). Zhou et al. (2020) conducted a study comparing the effects of varying levels of ammonium (0, 15, 25, and 35 ppm) on the growth of Arbor Acres broiler chickens from 22 days of age. At 36 and 43 days of age, a linear decline in chicken body weight was observed that corresponded to increasing levels of ammonia. The difference in live body weight at 43 days of age between the 0 ppm and 35 ppm groups was 2216 g and 1522 g, respectively. It is essential to maintain ammonia levels below 25 ppm at the end of fattening to comply with the growth curve. Litter type can influence chicken growth, as well. A meta-analysis conducted by Toledo et al. (2019) compared numerous litter materials utilized in various regions and their impact on chicken growth. The findings indicated that wood shavings were the most effective in promoting optimal growth. Some evidence suggests that the use of wood shavings as a litter material may result in reduced ammonia production compared to other materials, such as rice hulls, sand, and vermiculite (Miles et al., 2011). It is important to ensure that the moisture content of the litter is kept to a minimum, as this can lead to an increase in ammonia levels. De Jong et al. (2014) conducted a study in which they sprayed 300 ml of water per square meter of litter daily. They observed that the overall weight of the chickens decreased by 8% in the wetted litter group at 37 days of age compared to the control group, whose litter did not receive any added water. It is recommended that the moisture content of the litter be maintained at 20-25%. However, in a field study conducted in Italy (Meluzzi et al., 2008), moisture levels fluctuated seasonally between 32% and 50% during the winter, and between 25% and 39% in the summer. It is therefore evident that bedding during the fattening period is paramount in order to reduce the moisture content of the litter and the production of ammonia from the litter itself. Furthermore, a recent study has demonstrated that the structure of the litter has a significant impact on ammonia production. In this study, a friable litter structure was compared with compact litter that had a crust on the surface. The results demonstrated that the compact litter produced 49% less ammonia than the friable litter, which therefore provides strong rationale for crusted litter material (Brink et al., 2022). In light of the research above, it can be concluded that the condition of the litter in the final third of the fattening period plays a pivotal role in preventing hock burn, footpad dermatitis and lameness while ensuring optimal chicken growth.
Light program
Due to the high photosensitivity of birds, light plays a crucial role in chicken development. As such, farmers are able to use light programs to adjust the growth curve of a flock. In young chicks, a higher light intensity is used to stimulate feed intake and growth. However, in older chickens undergoing the fattening phase, increased light intensity above 30 lx is associated with significant negative impacts on chicken body weight (Yang et al., 2018). The optimal light intensity for ensuring optimal growth is 20 lx (Kim et al., 2022). Increasing the length of exposure leads to a linear increase in chicken body weight due to a stimulation of feed intake (Yang et al., 2015). Furthermore, different colors of light have been shown to yield different results. The use of green light resulted in a 6% increase in body weight at the end of the fattening period (42 days) compared to yellow and red light (Gharahveysi et al., 2020). Oke et al. (2020) conducted an interesting study comparing the effects of blue and green light with standard white light. A 54-day-old Arbor Acre broiler exposed to green light until 28 days of age and subsequently to blue light until the end of fattening had a final weight of 3.46 kg, which was significantly higher than those exposed to white light, with a final weight of 2.6 kg. Research thus indicates that It is essential to maintain a light regimen with an appropriate light intensity of approximately 20 lx and a light period of approximately 18-20 hours (Aviagen, 2010), with an equal distribution throughout the barn. Furthermore, the color of the light can be adjusted to stimulate increased growth in flocks with suboptimal development.
Leg problems
Modern fast-growing broiler chickens exhibit a number of significant health issues related to leg disorders, hock burn, and skin lesions. These conditions result in a worsened gait score, which in turn causes painful conditions and decreased movement . This, in turn, has deleterious effects on feed and water intake, which consequently affect growth. In the Netherlands, the quantified production costs per delivered broiler due to leg problems were 1.19%, which was the second highest number after most pronounced bacterial diseases (Gocsik et al., 2014). Conversely, chickens raised especially to higher weight normally have issues with walking ability due to the weight of their bodies and their redistributed center of gravity. Figure 1 illustrates the correlation between increased gait score (described in Table 1) and increased body weight in Cobb 500 broilers. Farmers can positively influence chicken leg health through flock management. One effective strategy is to decrease feed amounts per session while increasing feeding frequency. The result is increased feeding time and exercise levels for the broilers. Increased chicken activity is positively associated with leg health (Bizeray et al., 2002). It has been demonstrated that foot pad dermatitis and hock burns are highly correlated with higher litter moisture, with the peak incidence occurring after 21 days of age. In one study, an increased incidence of leg problems was observed at 28 days of age, later accompanied by a significant decline in body weight gain (20%) at 37 days of age (De Jong et al., 2014). A brief period of aggravated leg health at the conclusion of the fattening process may disrupt the outcome of the fattening. Therefore, it is crucial to select high-quality bedding to prevent bacterial growth, particularly mold contamination, which can lead to dermatitis in chickens. Appropriate litter management is highly correlated with achieving optimal growth.
| Gait score | Description |
| 0 | Chickens are normal, smooth, and agile. |
| 1 | Slight abnormality in walking, but negligible. |
| 2 | Definite and identifiable abnormality, but has limited impact on walking ability |
| 3 | Obvious abnormality affects the ability to move. The bird has imbalanced steps and squats within 15 s |
| 4 | Severe abnormality, but still capable of walking. The bird takes more than 5 s to rise when nudged, and squats after a few steps |
| 5 | Chickens are incapable of walking |
Tab. 1 Gait scoring system according to Yang et al. (2023)
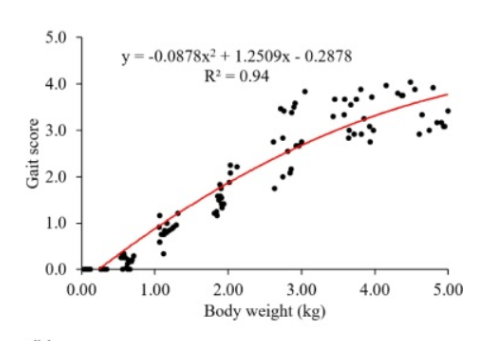
Figure 1 – Correlations of gait score and body weight for Cobb 500. A greater gait score means worse walking ability (Yang et al., 2023)
Coccidiosis
The most significant challenge impeding the growth of chickens is coccidiosis caused by the gastrointestinal parasite Eimeria.The annual revenue lost to diminished performance in chickens due to coccidiosis is approximately 11.93 billion dollars (Blake et al., 2020). The prevalence of coccidia on farms in Europe is approximately 65%, where they have a considerable impact on chicken growth (Gocsik et al., 2014). Meta-analyses conducted by Freitas et al. (2023) showed a reduction in daily feed intake of 19 g and an average daily gain of 39 g in chickens infected with Eimeria. The growth rate was found to be 10.5% lower in coccidia-infected chickens (Freitas et al., 2023). Coccidiosis can be mitigated through the use of coccidiostats in feed and vaccination. However, housing management remains the most important factor in suppressing coccidia infection. The multifactorial nature of coccidia prevention is beyond the scope of this article. Ahmad et al. (2024) provides a summary of the most important housing management factors that potentiate the growth of coccidia. The prevalence of coccidia is predominantly observed in the second half of the fattening period, due to the deterioration of litter quality, which provides an optimal environment for coccidia multiplication. As such, litter quality is a crucial factor in the prevention of coccidia. Effective methods for mitigating the prevalence of coccidia in a flock include lighting, ventilation, temperature control, and equipment design to reduce injury risk. During periods of stress, it is advisable to supplement electrolytes to maintain health. It is imperative to implement rigorous biosecurity measures to prevent the introduction and spread of disease. This entails maintaining a clean and safe environment for the chickens, which necessitates the use of appropriate attire and footwear by employees, as well as adherence to hygienic procedures such as handwashing and the cleaning and disinfection of tools and vehicles. Facility cleaning and disinfection, ventilation, and the provision of clean water can effectively maintain litter conditions and reduce oocysts. Prophylaxis, or the use of anticoccidials to prevent disease, represents a crucial aspect of broiler chicken production (Ahmad et al., 2024).
The growth of chickens is influenced by a multitude of factors, and this article presents the most crucial of them, along with recommendations for their optimization. The growth rate of chickens is a sensitive indicator of discomfort in a flock. Therefore, real-time, accurate measurement of the flock’s growth can be a tool for the initial identification of discrepancies in the aforementioned factors, which can assist farmers in implementing corrective measures to remedy the growth curve of the flock.
In closing, it is vital to remember that effective management practices like those mentioned above rely heavily on the accuracy and reliability of available bird weight data. Using specialized poultry weighing systems, such as BAT poultry scales, allows for well-informed decisions that maximize flock performance.
References
Ahmad, R., Yu, Y. H., Hua, K. F., Chen, W. J., Zaborski, D., Dybus, A., Hsiao, F. S., & Cheng, Y. H. (2024). Management and control of coccidiosis in poultry – A review. Animal bioscience, 37(1), 1–15. https://doi.org/10.5713/ab.23.0189
Aviagen, 2010. Lighting for broilers. available at: https://en.aviagen.com/assets/Uploads/RossTechLightingforBroilers.pdf
Baracho, M. S., Nääs, I. D. A., Lima, N. D. S., Cordeiro, A. F. S., & Moura, D. J. (2019). Factors affecting broiler production: A meta-analysis. Brazilian Journal of Poultry Science, 21, eRBCA-2019.
Bizeray, D., Estevez, I., Leterrier, C., & Faure, J. M. (2002). Influence of increased environmental complexity on leg condition, performance, and level of fearfulness in broilers. Poultry science, 81(6), 767–773. https://doi.org/10.1093/ps/81.6.767
Blake, D. P., Knox, J., Dehaeck, B., Huntington, B., Rathinam, T., Ravipati, V., … & Tomley, F. M. (2020). Re-calculating the cost of coccidiosis in chickens. Veterinary Research, 51, 1-14.
Brink, M., Janssens, G. P., & Delezie, E. (2022). How do moisture content, friability, and crust development of litter influence ammonia concentrations in broiler production?. Livestock Science, 265, 105109.
De Jong, I. C., Gunnink, H., & Van Harn, J. (2014). Wet litter not only induces footpad dermatitis but also reduces overall welfare, technical performance, and carcass yield in broiler chickens. Journal of Applied Poultry Research, 23(1), 51-58.
Freitas, L. F. V. B., Sakomura, N. K., Reis, M. P., Mariani, A. B., Lambert, W., Andretta, I., & Létourneau-Montminy, M. P. (2023). Coccidiosis infection and growth performance of broilers in experimental trials: insights from a meta-analysis including modulating factors. Poultry science, 102(11), 103021. https://doi.org/10.1016/j.psj.2023.103021
Gharahveysi, S., Irani, M., Kenari, T. A., & Mahmud, K. I. (2020). Effects of colour and intensity of artificial light produced by incandescent bulbs on the performance traits, thyroid hormones, and blood metabolites of broiler chickens. Italian Journal of Animal Science, 19(1), 1-7.
Gocsik, É., Kortes, H. E., Lansink, A. G., & Saatkamp, H. W. (2014). Effects of different broiler production systems on health care costs in the Netherlands. Poultry science, 93(6), 1301–1317. https://doi.org/10.3382/ps.2013-03614
Hulet, R., Gladys, G., Hill, D., Meijerhof, R., & El-Shiekh, T. (2007). Influence of egg shell embryonic incubation temperature and broiler breeder flock age on posthatch growth performance and carcass characteristics. Poultry science, 86(2), 408–412. https://doi.org/10.1093/ps/86.2.408
Iqbal, J., Khan, S. H., Mukhtar, N., Ahmed, T., & Pasha, R. A. (2016). Effects of egg size (weight) and age on hatching performance and chick quality of broiler breeder. Journal of Applied Animal Research, 44(1), 54–64. https://doi.org/10.1080/09712119.2014.987294
Kim, H. J., Son, J., Kim, H. S., Hong, E. C., & Kim, J. H. (2022). Effects of light intensity on growth performance, blood components, carcass characteristics, and welfare of broilers. Journal of animal science and technology, 64(5), 985–996. https://doi.org/10.5187/jast.2022.e47
Liu, K. L., He, Y. F., Xu, B. W., Lin, L. X., Chen, P., Iqbal, M. K., Mehmood, K., & Huang, S. C. (2023). Leg disorders in broiler chickens: a review of current knowledge. Animal biotechnology, 34(9), 5124–5138. https://doi.org/10.1080/10495398.2023.2270000
Liu, L., Ren, M., Ren, K., Jin, Y., & Yan, M. (2020). Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poultry science, 99(11), 6205–6211. https://doi.org/10.1016/j.psj.2020.08.019
Meluzzi, A., Fabbri, C., Folegatti, E., & Sirri, F. (2008). Survey of chicken rearing conditions in Italy: effects of litter quality and stocking density on productivity, foot dermatitis and carcase injuries. British Poultry Science, 49(3), 257–264. https://doi.org/10.1080/00071660802094156
Miles, D. M., Branton, S. L., & Lott, B. D. (2004). Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poultry science, 83(10), 1650-1654.
Miles, D. M., Rowe, D. E., & Cathcart, T. C. (2011). Litter ammonia generation: moisture content and organic versus inorganic bedding materials. Poultry science, 90(6), 1162–1169. https://doi.org/10.3382/ps.2010-01113
Oke, O. E., Oni, A. I., Adebambo, P. O., Oso, O. M., Adeoye, M. M., Lawal, T. G., … & Smith, O. F. (2021). Evaluation of light colour manipulation on physiological response and growth performance of broiler chickens. Tropical Animal Health and Production, 53, 1-9.
Son, J., Kim, H. J., Hong, E. C., & Kang, H. K. (2022). Effects of stocking density on growth performance, antioxidant status, and meat quality of finisher broiler chickens under high temperature. Antioxidants, 11(5), 871.
Toledo, T. D. S. D., Pich, C. S., Roll, A. A. P., Dai Prá, M. A., Leivas Leite, F., Gonçalves Xavier, E., & Roll, V. F. B. (2019). The effect of litter materials on broiler performance: a systematic review and meta-analysis. British poultry science, 60(6), 605-616.
Tona, K., Onagbesan, O., De Ketelaere, B., Decuypere, E., & Bruggeman, V. (2004). Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight, and chick posthatch growth to forty-two days. Journal of Applied Poultry Research, 13(1), 10-18.
Ulmer-Franco, A. M., Fasenko, G. M., & O’Dea Christopher, E. E. (2010). Hatching egg characteristics, chick quality, and broiler performance at 2 breeder flock ages and from 3 egg weights. Poultry science, 89(12), 2735–2742. https://doi.org/10.3382/ps.2009-00403
van der Eijk, J. A. J., van Harn, J., Gunnink, H., Melis, S., van Riel, J. W., & de Jong, I. C. (2023). Fast- and slower-growing broilers respond similarly to a reduction in stocking density with regard to gait, hock burn, skin lesions, cleanliness, and performance. Poultry science, 102(5), 102603. https://doi.org/10.1016/j.psj.2023.102603
Willemsen, H., Everaert, N., Witters, A., De Smit, L., Debonne, M., Verschuere, F., Garain, P., Berckmans, D., Decuypere, E., & Bruggeman, V. (2008). Critical assessment of chick quality measurements as an indicator of posthatch performance. Poultry science, 87(11), 2358–2366. https://doi.org/10.3382/ps.2008-00095
Yahav, S., Straschnow, A., Vax, E., Razpakovski, V., & Shinder, D. (2001). Air velocity alters broiler performance under harsh environmental conditions. Poultry science, 80(6), 724–726. https://doi.org/10.1093/ps/80.6.724
Yang, X., Zhao, Y., Gan, H., Hawkins, S., Eckelkamp, L., Prado, M., Burns, R., Purswell, J., & Tabler, T. (2023). Modeling gait score of broiler chicken via production and behavioral data. Animal : an international journal of animal bioscience, 17(1), 100692. https://doi.org/10.1016/j.animal.2022.100692
Yang, Y. F., Jin, S. F., Zhong, Z. T., Yu, Y. H., Yang, B., Yuan, H. B., & Pan, J. M. (2015). Growth responses of broiler chickens to different periods of artificial light. Journal of animal science, 93(2), 767–775. https://doi.org/10.2527/jas.2014-8096
Yang, Y., Pan, C., Zhong, R., & Pan, J. (2018). Artificial light and biological responses of broiler chickens: dose-response. Journal of animal science, 96(1), 98–107. https://doi.org/10.1093/jas/skx044
Yi, B., Chen, L., Sa, R., Zhong, R., Xing, H., & Zhang, H. (2016). Transcriptome profile analysis of breast muscle tissues from high or low levels of atmospheric ammonia exposed broilers (Gallus gallus). PLoS One, 11(9), e0162631.
Zhou, Y., Liu, Q. X., Li, X. M., Ma, D. D., Xing, S., Feng, J. H., & Zhang, M. H. (2020). Effects of ammonia exposure on growth performance and cytokines in the serum, trachea, and ileum of broilers. Poultry science, 99(5), 2485–2493. https://doi.org/10.1016/j.psj.2019.12.063
