The quality of day-old chicks represents a critical factor in determining profitability. Farmers face considerable challenges in influencing this factor, as chick quality is established at very early developmental stages. Upon arrival at the farm, farmers must be able to identify substandard chicks and understand all factors affecting day-old chick quality. This knowledge enables them to pinpoint problem sources and implement necessary corrections. This article focuses on assessing day-old chick quality and provides information about factors that influence chick quality.
How to determine chick quality
When selecting high-quality chicks, several key physical attributes should be observed: their eyes should be bright and alert, indicating good health and vitality; the body should be free from deformities or injuries; the navel should be completely closed with fully retracted yolk, signifying proper development and reduced infection risk; the chick should be completely separated from any remaining membrane or shell; it should readily react to stimuli, demonstrating alertness and sound neurological function; there should be no signs of edema or swelling on the body, which could indicate underlying health problems; and finally, the chick should appear alert and interested in its environment, suggesting overall vigor and well-being (Narinç and Aydemir, 2021).
Traditional quantitative methods for assessing chick quality include chick weight and chick length; however, these parameters have limitations when used alone.
Chick weight alone can be misleading as a quality indicator because it includes residual yolk, which isn’t directly related to quality.
The strong positive correlation between egg weight and chick weight further complicates using chick weight as a sole measure.
Yolk-free body weight is proposed as a more accurate quality measure, showing positive relationships with subsequent bird performance.
Chick length is also associated with yolk-free body weight and can predict future chick performance.
Therefore, additional qualitative parameters are recommended, as they more accurately describe current chick quality status.
Tona scoring qualitative method to determine chick quality
The Tona scoring method comprises several parameters that comprehensively describe chicks (Tona et al., 2003). It consists of multiple parameters described by scoring scales based on visual examination (Figure 1).
- Chick activity is evaluated by observing the time required for a chick to right itself after being placed on its back. Rapid return to an upright position indicates strong activity, while slow or failed attempts to right itself suggest weakness.
- Dryness and cleanliness: A chick is considered normal if its body is dry and clean. Conversely, a wet or dirty chick, or one that is both wet and dirty (potentially leading to contamination), is deemed poor quality.
- Yolk retraction: The chick is gently placed on its back at an angle on the palm of a hand and observed until abdominal movement ceases completely. At this point, the height of its abdomen is visually assessed, followed by evaluation of its consistency to the touch. An abdomen that appears higher and feels firmer than normal indicates large and consistent yolk retraction.
- Eye condition: The chick is placed upright on its legs, and its eyes are carefully examined. The brightness and degree to which its eyelids are open (gape) are assessed.
- Leg quality: To assess the chick’s ability to stand, it is placed on its feet. The conformation of its toes is then inspected. If the chick struggles to remain upright, the knee joints are examined for any indications of inflammation or redness.
- The navel and adjacent areas are inspected for complete closure and any discoloration. A navel exhibiting different coloration from the chick’s skin tone indicates poor quality.
- Remaining yolk: By examining the navel, the extent of residual yolk can be assessed and classified into three categories: very large, large, or small.
- Membrane size: Observation of the navel area allows estimation of any remaining membrane size. The remaining membrane size is classified as very large, large, or small.
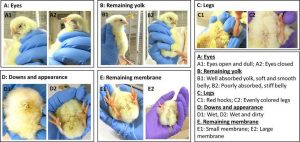
Figure 1 – Several examples of parameters evaluated by the Tona scoring method according to Gao et al. (2024)
Identification of problems with chick quality
This section focuses on identifying factors that contribute to substandard chick quality. It is important to emphasize that these issues manifest before chicks arrive at farms. Consequently, effective communication between all production system elements is paramount: farms, transportation, hatcheries, and breeder farms. Lack of effective communication and information exchange can impede chick production and quality optimization. This section examines key factors that influence chick quality, with reference to parent stocks, egg storage, hatcheries, and post-hatch handling.
Breeder flocks
Impact of parent stock age
- Chick weight increases with breeder bird age. Quantitative methods using chick weight as a measure might incorrectly suggest that higher quality chicks come from older hens (Tona et al., 2005).
- Albumen quality deteriorates with increasing breeder age, leading to regression in chick quality (Fasenko et al., 1992).
- Several factors contribute to declining chick quality from older breeder flocks, including deterioration of eggshell quality, increased water loss during incubation, aging of oocytes, changes in yolk composition, and shortening of the laying sequence (Narinç and Aydemir, 2021).
- Younger breeder flocks generally produce higher quality chicks as assessed by qualitative methods (e.g., Tona score, Pasgar score, anatomical defects, navel appearance) (Tona et al., 2004).
- Studies using qualitative methods consistently show negative correlations between breeder age and chick quality scores. This is observed even after egg storage.
- Studies using quantitative methods (chick weight and length) generally show positive correlations between breeder age and these physical attributes. This can lead to conflicting interpretations of chick quality.
Impact of parent stock nutrition
- While egg energy and protein composition remain stable, dietary protein in the breeder flock significantly impacts progeny performance, particularly breast meat yield in male offspring. Very low protein can negatively affect egg and chick weight (Lopez and Leeson, 1995).
- Mineral and vitamin content in the layer diet on the parent farm is critical for normal chick embryo development and day-old chick quality. Specifically, vitamins D and E, selenium, zinc, and magnesium are important.
- Vitamin D3 levels in the layer diet are linked to vitamin D3 in egg yolk. Optimal vitamin D3 in the diet can improve eggshell quality, hatchability, chick quality, offspring growth, and innate immunity (Matilla et al., 1999).
- Vitamin E and selenium levels in eggs can be increased by adjusting broiler breeder hen diets.
- Supplementing hen feed with 60 IU/kg of vitamin E led to significantly higher antibody levels in day-old chicks, suggesting enhanced immunity (Andi et al., 2006).
- Increased transfer of maternal antibodies due to vitamin E supplementation in hens results in stronger immune responses in their offspring (Andi et al., 2006).
- Proper antibody production after hatch is crucial for disease resistance; therefore, parent stock nutrition can highly influence disease susceptibility and early mortality in their progeny.
Preincubation conditions (egg storage)
- Optimal Storage Temperature: Decuypere et al. (2001) suggest a storage temperature of 13–17°C.
- Physiological Zero Point: The concept of a physiological zero point (where embryonic development slows or stops) is crucial for determining storage conditions. This point is reported to be in the range of 19–28°C and below.
- Storage Duration Impact: Ideally, egg storage should not exceed 7 days. Longer storage periods generally decrease hatchability and chick quality (Gharib, 2013). Chick weight may be reduced with longer storage (Tona et al., 2004). Short-term storage (e.g., 3-5 days) might have positive effects on chick length (Reijrink et al., 2009). Navel quality might be negatively affected by longer storage periods above 7 days (Reijrink et al., 2010).
- Albumen Quality Degradation: Water and carbon dioxide loss during storage leads to increased albumen acidity, damaging beneficial proteins and antimicrobial entities, which can negatively impact embryo development and defense against pathogens.
- Breeder Flock Age: Breeder flock age influences storage impact. Eggs from older flocks are more susceptible to quality reduction during storage compared to eggs from younger flocks (Damaziak et al., 2018).
- Relative Humidity (RH): Optimal humidity at storage can be recommended based on Damaziak et al. (2018) at 65–75% because this range yields highest chick quality.
- Preheating: Preheating eggs before incubation under specific storage conditions (15–18°C and 65–75% RH for 5 and 12 days) from older flocks showed the highest Tona scores (Damaziak et al., 2018), suggesting it can mitigate some negative effects of storage.
Hatcheries (incubation conditions)
- Temperature, humidity, and air circulation are essential and optimally controlled in modern commercial poultry houses using automatic incubation systems.
- The optimal incubation temperature range for domestic poultry is narrower (37-38°C) than for wild chickens (33-39°C).
- Maintaining incubation temperature within the optimum range is essential for normal embryonic development, successful hatching, chick quality, and post-hatching performance.
- High incubation temperatures negatively impact embryonic development, shorten the incubation period, and increase mortality and post-hatching abnormalities, especially during the hatching period (Narinç and Aydemir, 2021).
- Low incubation temperatures can decrease embryo weight and the rate of high-quality chicks (Narinç and Aydemir, 2021).
- While constant eggshell temperature of 37.5-38.0°C is generally recommended, some studies suggest slightly higher temperature in early stages followed by 37.5-38.0°C from day 9 can improve hatchability and chick quality (Lourens et al., 2005).
- Eggs lose water and weight through evaporation during incubation.
- Humidity in the incubator is directly related to the egg’s water loss.
- Optimal humidity for setting compartments is recommended at 55% and for hatching compartments at range from 65–90%.
- Multiple studies (Molenaar et al. 2013; Van der Pol et al. 2013) have shown that non-optimal humidity levels are detrimental to chick quality.
- Water loss (11-13% being optimum) through evaporation during incubation directly affects chick quality; losses outside this range are detrimental.
- The first week of incubation is most critical for gas exchange due to the distance between the embryo and shell and high albumen density. Gas exchange relies on diffusion initially.
- Carbon dioxide (CO2) levels influence chick weight and quality (Bruggeman et al., 2006).
- Elevated levels (1%) of CO2 until 10 embryonic day and in the last 3 days of incubation can positively influence chick quality and shorten hatching windows (Liu et al., 2022).
- Oxygen (O2) levels also impact chick quality. Increased O2 levels (up to 25%) can increase chick length and weight, thus improving quality. However, interaction with temperature is important, as 25% O2 at high temperature (38.9°C) increased chick length but reduced weight (Lourens et al., 2007).
- Egg turning during incubation mimics natural poultry behavior.
- Turning facilitates gas exchange, prevents dehydration (despite water loss), and is crucial for proper embryonic fluid and ligament formation and nutrient absorption, as well as overall embryonic growth (Elibol and Brake, 2006).
- For normal embryonic development, eggs should be turned at a 90° angle, similar to natural incubation (Elibol and Brake, 2006).
Post hatch handling and transportation
- Chick quality is mainly influenced by post-hatch handling.
- Post-hatch treatment includes sorting, vaccination, transportation, and placement.
- Delayed placement time and feed fasting after hatching have negative effects on chick performance.
- Researchers reported that 36 h feed and water deprivation after hatching had negative effects on performance (Özlü et al., 2020).
- Day-old chick transportation represents the weakest point.
- Journey duration and relative humidity (RH) during commercial transportation impact the body weight of day-old chicks (Yerpes et al., 2020).
- Transportation Duration: While longer transportation (11 hours) temporarily reduced yolk size and body weight of day-old chicks compared to shorter transportation, this weight difference did not persist until slaughter age. Importantly, transportation duration (up to 11 hours) had no significant impact on chick quality, mortality, or meat quality (Jacobs et a., 2016).
- Parent stock age has no influence on chick quality in interaction with transportation (Jacobs et a., 2016).
- Based on literature, stocking density in transport boxes below 26.7 cm²/per chick can be recommended to prevent overcrowding and creation of heat spots with detrimental effects on chick quality (Jhetam et al., 2024).
- Transportation impacts are highly influenced by season, in other words by environmental conditions, which highly influence study results; therefore, contradictory results are obtained regarding transportation effects on day-old chick quality.
Conclusion
Mass production of day-old chicks creates a complex production system in which a single weak link can compromise the outcome. Compensatory growth in chicks has been demonstrated to occur in response to adverse conditions. However, it is important to acknowledge that chick quality is significantly associated with seven-day mortality rates, with detrimental consequences following. Farmers can evaluate their chick quality using both quantitative and qualitative methods and provide feedback to hatcheries and breeder farms. Conversely, hatcheries must furnish farmers with pertinent information (e.g., parent stock age, egg storage duration, hatchery discrepancies, and post-hatch handling). This information is crucial for farmers‘ expectations of their broilers‘ growth potential. Nevertheless, information dissemination between various components of day-old chick production is frequently inadequate.
References
Andi, M. A., Shivazad, M., Pourbakhsh, S. A., Afshar, M., Rokni, H., Shiri, N. E., … & Salahi, Z. (2006). Effects of vitamin E in broiler breeder diet on hatchability, egg quality and breeder and day old chick immunity.
Bruggeman, V., Smit, L. D., Tona, K., Everaert, N., Witters, A., Debonne, M., … & Decuypere, E. (2006). Changes in albumen pH due to higher CO2 concentrations during the first ten days of incubation.
Damaziak, K., Pawęska, M., Gozdowski, D., & Niemiec, J. (2018). Short periods of incubation, egg turning during storage and broiler breeder hens age for early development of embryos, hatching results, chicks quality and juvenile growth. Poultry Science, 97(9), 3264-3276.
Decuypere, E., Tona, K., Bruggeman, V., & Bamelis, F. (2001). The day-old chick: a crucial hinge between breeders and broilers. World’s Poultry Science Journal, 57(2), 127-138.
Elibol, O. K. A. N., & Brake, J. (2006). Effect of egg turning angle and frequency during incubation on hatchability and incidence of unhatched broiler embryos with head in the small end of the egg. Poultry science, 85(8), 1433-1437.
Fasenko, G. M., Hardin, R. T., Robinson, F. E., & Wilson, J. L. (1992). Relationship of hen age and egg sequence position with fertility, hatchability, viability, and preincubation embryonic development in broiler breeders. Poultry science, 71(8), 1374-1383.
Gao, M., Ren, Y., Lu, S., Reddyvari, R., Venkitanarayanan, K., & Amalaradjou, M. A. (2024). In ovo probiotic supplementation supports hatchability and improves hatchling quality in broilers. Poultry science, 103(6), 103624. https://doi.org/10.1016/j.psj.2024.103624
Gharib, H. (2013). Effect of pre-storage heating of broiler breeder eggs, stored for long periods, on hatchability and chick quality. Egyptian Journal of Animal Production, 50(3), 174-184.
Jacobs, L., Delezie, E., Duchateau, L., Goethals, K., Ampe, B., Lambrecht, E., … & Tuyttens, F. A. (2016). Effect of post-hatch transportation duration and parental age on broiler chicken quality, welfare, and productivity. Poultry Science, 95(9), 1973-1979.
Jhetam, S., Shynkaruk, T., Buchynski, K., Van Kessel, A. G., Crowe, T. G., & Schwean-Lardner, K. (2024). Stocking density within chick transport boxes: effects on leghorn chick stress and box microclimate. Journal of Applied Poultry Research, 33(2), 100400.
Liu, C., Zheng, W., Zhu, L., Tong, Q., & Li, D. (2022). Effect of elevated carbon dioxide on chicken eggs during the early and late incubation periods. Animal, 16(4), 100499.
LOPEZ, G., & LEESON, S. (1995). Response of broiler breeders to low-protein diets.: 2. Offspring performance. Poultry Science, 74(4), 696-701.
Lourens, A., Van den Brand, H., Heetkamp, M. J. W., Meijerhof, R., & Kemp, B. (2007). Effects of eggshell temperature and oxygen concentration on embryo growth and metabolism during incubation. Poultry science, 86(10), 2194-2199.
Lourens, A., Van den Brand, H., Meijerhof, R., & Kemp, B. (2005). Effect of eggshell temperature during incubation on embryo development, hatchability, and posthatch development. Poultry science, 84(6), 914-920.
Mattila, P., Lehikoinen, K., Kiiskinen, T., & Piironen, V. (1999). Cholecalciferol and 25-hydroxycholecalciferol content of chicken egg yolk as affected by the cholecalciferol content of feed. Journal of agricultural and food chemistry, 47(10), 4089–4092. https://doi.org/10.1021/jf990183c
Molenaar, R., van den Borne, J. J., Hazejager, E., Kristensen, N. B., Heetkamp, M. J., Meijerhof, R., … & van den Brand, H. (2013). High environmental temperature increases glucose requirement in the developing chicken embryo. PLoS One, 8(4), e59637.
Narinç, D. O. Ğ. A. N., & Aydemir, E. (2021). Chick quality: an overview of measurement techniques and influencing factors. World’s Poultry Science Journal, 77(2), 313-329.
Özlü, S. E. R. D. A. R., Uçar, A. H. M. E. T., Romanini, C. E. B., Banwell, R., & Elibol, O. K. A. N. (2020). Effect of posthatch feed and water access time on residual yolk and broiler live performance. Poultry science, 99(12), 6737-6744.
Reijrink, I. A. M., Meijerhof, R., Kemp, B., Graat, E. A. M., & Van den Brand, H. (2009). Influence of prestorage incubation on embryonic development, hatchability, and chick quality. Poultry Science, 88(12), 2649-2660.
Reijrink, I. A. M., Van Duijvendijk, L. A. G., Meijerhof, R., Kemp, B., & Van Den Brand, H. (2010). Influence of air composition during egg storage on egg characteristics, embryonic development, hatchability, and chick quality. Poultry Science, 89(9), 1992-2000.
Tona, K., Bamelis, F. B. V. V. M. J. O. E., De Ketelaere, B., Bruggeman, V., Moraes, V. M., Buyse, J., … & Decuypere, E. (2003). Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poultry science, 82(5), 736-741.
Tona, K., Onagbesan, O. M., Jego, Y., Kamers, B., Decuypere, E., & Bruggeman, V. (2004). Comparison of embryo physiological parameters during incubation, chick quality, and growth performance of three lines of broiler breeders differing in genetic composition and growth rate. Poultry science, 83(3), 507-513.
Tona, K., Onagbesan, O. M., Jego, Y., Kamers, B., Decuypere, E., & Bruggeman, V. (2004). Comparison of embryo physiological parameters during incubation, chick quality, and growth performance of three lines of broiler breeders differing in genetic composition and growth rate. Poultry science, 83(3), 507-513.
Tona, K., Onagbesan, O., Bruggeman, V., Mertens, K., & Decuypere, E. (2005). Effects of turning duration during incubation on embryo growth, utilization of albumen, and stress regulation. Poultry Science, 84(2), 315-320.
Van der Pol, C. W., van Roovert-Reijrink, I. A. M., Maatjens, C. M., Van den Brand, H., & Molenaar, R. (2013). Effect of relative humidity during incubation at a set eggshell temperature and brooding temperature posthatch on embryonic mortality and chick quality. Poultry Science, 92(8), 2145-2155.
Yerpes, M., Llonch, P., & Manteca, X. (2020). Effect of environmental conditions during transport on chick weight loss and mortality. Poultry science, 100(1), 129.
